Abstract
This study investigates the neurophysiological aspects of Zen meditation (zazen) through a single-subject, longitudinal design. Electroencephalographic (EEG), heart rate, and movement data are collected using the Muse 2 headband in daily 30-minute meditation sessions. By examining the shifts in neural oscillations across frequency bands, this research seeks to reveal the neural correlates of zazen and contribute to understanding of meditation-induced neuroplastic changes.
Introduction ↑
Zen meditation, or “zazen” (seated meditation), is a practice aimed at cultivating heightened awareness, mental clarity, and a detachment from discursive thought. Practitioners use an open-awareness approach to reach a state of “no mind” or non-conceptual presence.
Meditation involves transitioning from thought-driven activity to neurophysiological states associated with focused attention, relaxation, and non-dual awareness. Neural oscillations, which synchronize and integrate cognitive processes across brain regions, serve as a useful framework to study these states. Meditation may modulate neural oscillations by reducing activity in the default mode network (DMN), a system active during passive rest and mind-wandering but suppressed during focused tasks. Prior research suggests experienced meditators exhibit decreased DMN activity, which aligns with reductions in self-referential thinking and increased gamma oscillations, believed to facilitate neural integration during meditation .
More research is needed on differences in neural correlates across meditative traditions, especially as they pertain to long-term changes rather than brief states. This study aims to advance the neuroscientific study of meditation by focusing specifically on Zen meditation and collecting extended time-series data.
Hypotheses ↑
This study tests three primary hypotheses:
1. Oscillatory dynamics: Daily zazen practice will produce changes in neural oscillatory patterns, with expected increases in theta and alpha band power, reductions in beta power, and synchronization of gamma waves in deep meditative states.
2. Temporal progression: Neural oscillatory patterns will show progressive adaptation and stabilization across sessions, suggesting persistent neuroplastic changes.
3. Frequency band interaction: Inter-frequency coupling will display unique configurations throughout meditation stages, reflecting shifts in attentional and conscious states.
Literature review ↑
The scientific exploration of meditation’s effects on the brain has developed significantly since the pioneering EEG studies of the 1960s, which established basic links between meditation and neural oscillatory patterns . Kasamatsu and Hirai’s (1966) study on Zen practitioners marked a foundational moment in meditation research, documenting elevated alpha wave activity. This finding pointed to a unique state of relaxed alertness, distinct from typical wakefulness, that could be consistently measured. Wallace (1970) built on this work by investigating the distinct neurophysiological patterns of transcendental meditation compared to sleep and relaxation, suggesting the uniqueness of meditation as a neural state .
Research has broadened since these early studies to explore how various meditation practices influence distinct oscillatory patterns in neural networks:
- Delta oscillations (0.5-4 Hz), typically linked to deep sleep, have in most cases of (non-drowsy) meditation shown a decrease in power .
- Theta oscillations (4-8 Hz), associated with emotional processing, memory, and attention, are often heightened during meditation, particularly in frontal and midline regions, which may indicate internally directed attention and emotional regulation. Aftanas and Golocheikine (2001) found that enhanced frontal theta activity correlated with both meditation experience and the depth of meditative state, positing theta as a potential bridge between attention regulation and heightened awareness in meditation . Takahashi et al. (2005) demonstrated that Zen practitioners exhibit elevated theta activity in frontal brain regions, suggesting decreased attentional demands and a heightened inward focus .
- Alpha oscillations (8-12 Hz), generally linked to relaxed, open awareness, consistently increase during Zen meditation, particularly in experienced practitioners. This increase reflects a disengagement from external sensory input and a deepened internal focus. Early studies by Kasamatsu and Hirai (1966) and Wallace (1970) showed that long-term practitioners display sustained alpha activity, even with eyes open . More recent work is mixed. Some studies support these findings, suggesting that higher alpha activity in experienced meditators reflects their ability to regulate attention by disengaging from external distractions while enhancing focus on internal states , while others show reduced alpha power , at least in some meditative traditions.
- Beta oscillations (13-30 Hz), associated with cognitive processing and goal-directed thinking, exhibit distinct changes during Zen meditation. While beta activity generally indicates active engagement, focused attention, and sensory processing, meditation appears to modulate beta waves, especially during initial concentration phases, when focus is actively maintained. Sustained meditation can decrease beta activity over time, potentially indicating a transition from goal-oriented, analytical thinking to a more receptive, non-evaluative state .
- Gamma oscillations (30-100 Hz), often linked to high-level cognitive functions and conscious perception, show significant changes in experienced meditators across various practices, including Zen. Lutz et al. (2004) demonstrated increased gamma power and coherence in long-term meditators, suggesting enhanced integration across neural networks . This phenomenon, especially prominent in non-referential awareness practices like Zen, may correspond to the dissolution of egoic boundaries and a unified experience of consciousness reported by practitioners. Subsequent studies show that elevated gamma activity correlates with self-reported states of insight and connectedness, likely due to gamma’s role in facilitating synaptic communication and interregional coherence in the brain . This reduction in self-referential processing aligns with Zen’s goal of non-dual awareness, or the dissolution of the boundary between self and external reality.
The default mode network (DMN), which is active during self-referential thought and mind-wandering, has emerged as a significant focus in meditation research. Brewer et al. (2011) showed that advanced meditators exhibit diminished DMN activation, a finding supported by Berkovich-Ohana et al. (2012), who noted an increase in gamma power associated with reduced DMN activity . Enhanced connectivity between the DMN and other networks, such as the salience network, may allow meditators to alternate between inward-focused and outward-focused states, leading to increased cognitive flexibility and attentional regulation. This neural flexibility may underlie some of the attention-related benefits often reported by Zen practitioners. In addition, recent technological advancements, including high-density EEG, fMRI, and resting-state functional connectivity analyses, have suggested that regular meditation can lead to lasting neuroplastic changes, increasing connectivity within attentional networks, including the frontoparietal network and the dorsal attention network .
In sum, Zen meditation significantly influences neural oscillatory dynamics, especially in the alpha, theta, and gamma bands. High-frequency gamma oscillations, enhanced in experienced practitioners, may reflect the integration of sensory and cognitive processes, contributing to the subjective experience of insight and unity often associated with Zen practice. These neuroplastic effects, evidenced by longitudinal studies, highlight Zen meditation’s potential for long-term impacts on brain function and well-being.
Study design ↑
Methodology ↑
The study will collect neurophysiological data from daily 30-minute zazen sessions to examine neural oscillatory patterns using a Muse 2 headset. The device, equipped with five dry electrodes positioned according to the international 10-20 system (TP9, TP10, AF7, AF8, and FpZ; see “Hardware” section below), allows for continuous EEG recording. This configuration targets relevant brain regions, enabling measurement across alpha, beta, delta, gamma, and theta frequency bands.
During each session, raw EEG data is collected, capturing fluctuations across these frequency bands, while concurrent motion data helps control for artifacts associated with head movements. The meditation sessions are segmented into three intervals of ten minutes each, providing a basis for examining oscillatory dynamics over time. Additionally, subjective experience questionnaires are completed before and after each session to document phenomenological changes.
Hardware ↑
The data above was collected using the Muse 2 headset and the Mind Monitor app. The headset has five dry electrodes located at locations TP9, TP10, AF7, AF8, and FpZ, which refer to the international 10-20 system. Letters correspond to the pre-frontal (Fp), frontal (F), temporal (T), parietal (P), occipital (O), and central (C) brain regions. Numbers correspond to hemisphere: the left hemisphere of the brain is given even numbers, the right hemisphere odd numbers, and the midline sagittal plane of the skull—the line connecting just above the nose (“nasion”) to the back of the skull (“inion”)—is labeled “z” for zero.

Figure 1 shows the location of the Muse 2 electrodes; note that Fpz serves as the reference electrode, placed in a neutral location with respect to hemispheric activity. The other electrodes measure the differences in electrical potential between FpZ and their locations. The Muse 2 electrodes are focused on activity in the temporal, parietal, and frontal lobes.
Data descriptions ↑
TimeStamp, Date
Year-month-day and hour-minute-second of data point.
Delta
Mean value across all channels within the delta frequency band, measured in the logarithm of the sum of the Power Spectral Density (in bels) over the delta band. Delta waves (0.5-4 Hz) are the slowest brain waves and are typically associated with deep, dreamless sleep. However, during meditation, increased delta power has been observed, which may indicate a state of deep relaxation and internalized attention. The mean value is calculated from the absolute band power of Delta_AF7, Delta_AF8, Delta_TP9, and Delta_TP10 electrodes (see Figure 1).
Theta
Mean value across all channels within the theta frequency band, measured in the logarithm of the sum of the Power Spectral Density (in bels) over the theta band. Theta waves (4-8 Hz) are linked to states of deep relaxation, memory recall, and emotional processing. Numerous studies have found increases in theta power during meditation, particularly in frontal and midline regions of the brain, although oscillations in the theta band also occur during daydreaming and dreaming. An increase in theta activity may reflect a state of heightened awareness, internalized attention, and access to subconscious information. The mean value is calculated from the Theta_AF7, Theta_AF8, Theta_TP9, and Theta_TP10 electrodes (see Figure 1).
Alpha
Mean value across all channels within the alpha frequency band, measured in the logarithm of the sum of the Power Spectral Density (in bels) over the alpha band. Alpha waves (8-13 Hz) are associated with a state of relaxed wakefulness and mindful awareness, especially with eyes closed. Studies have reported increases in alpha power during meditation, especially in posterior regions of the brain. The enhancement of alpha activity may indicate a reduction in external sensory processing and a shift towards internalized, focused attention. The mean value is calculated from the Alpha_AF7, Alpha_AF8, Alpha_TP9, and Alpha_TP10 electrodes (see Figure 1).
Beta
Mean value across all channels within the beta frequency band, measured in the logarithm of the sum of the Power Spectral Density (in bels) over the beta band. Beta waves (13-32 Hz) are typically linked to active cognitive processing, problem-solving, and outward-directed attention. During meditation, beta power often decreases, which may reflect a reduction in analytical, goal-oriented thinking and a shift towards a more receptive, non-dual state of awareness. The mean value is calculated from the Beta_AF7, Beta_AF8, Beta_TP9, and Beta_TP10 electrodes (see Figure 1).
Gamma
Mean value across all channels within the gamma frequency band, measured in the logarithm of the sum of the Power Spectral Density (in bels) over the gamma band. Gamma waves (32-100 Hz) are associated with higher-order cognitive functions, such as perceptual binding, conscious perception, and heightened states of awareness. Some studies have found stronger and less variable gamma waves during meditation, particularly in experienced practitioners, which may indicate a state of enhanced sensory integration, conscious presence, and expanded self-awareness. The mean value is calculated from the Gamma_AF7, Gamma_AF8, Gamma_TP9, and Gamma_TP10 electrodes (see Figure 1).
RAW_AF7, RAW_AF8, RAW_TP9, RAW_TP10
Raw electroencephalogram data on each electrode channel, in microvolts (µV). Range between 0-1682.815 µV.
Accelerometer_X, Accelerometer_Y, Accelerometer_Z
Acceleration relative to gravity in the x (forward/back, i.e., head tilt up/down), y (head tilt left/right), and z (vertical motion up/down) directions, in milli-Gs (weight per unit mass, or acceleration vector). See Figure 2 below.
Gyro_X, Gyro_Y, Gyro_Z
Rotation from last headset position in the x (roll, i.e., positive when head tilts to right), y (pitch; positive when looking up), and z (yaw; positive when head turns to right) dimensions, measured in degrees per second. See Figure 2 below. Heart_Rate: Beats per minute, estimated using the PPG_Ambient, PPG_IR, and PPG_Red photoplethysmography variables.
HeadBandOn
A binary 0/1 variable indicating that the headset is being worn.
HSI_AF7, HSI_AF8, HSI_TP9, HSI_TP10
Horseshoe indicator measuring data quality for each sensor (“1” = “good”; “2” = “medium”; “3” = “bad”). ↑

Limitations ↑
This study’s single-subject design inherently limits its generalizability. The results will primarily reflect individual variability, which may not represent broader meditation practices. Additionally, the Muse 2 headband, while effective for general EEG data, lacks the clinical-grade precision of laboratory-grade equipment, potentially influencing the sensitivity of oscillatory measurements.
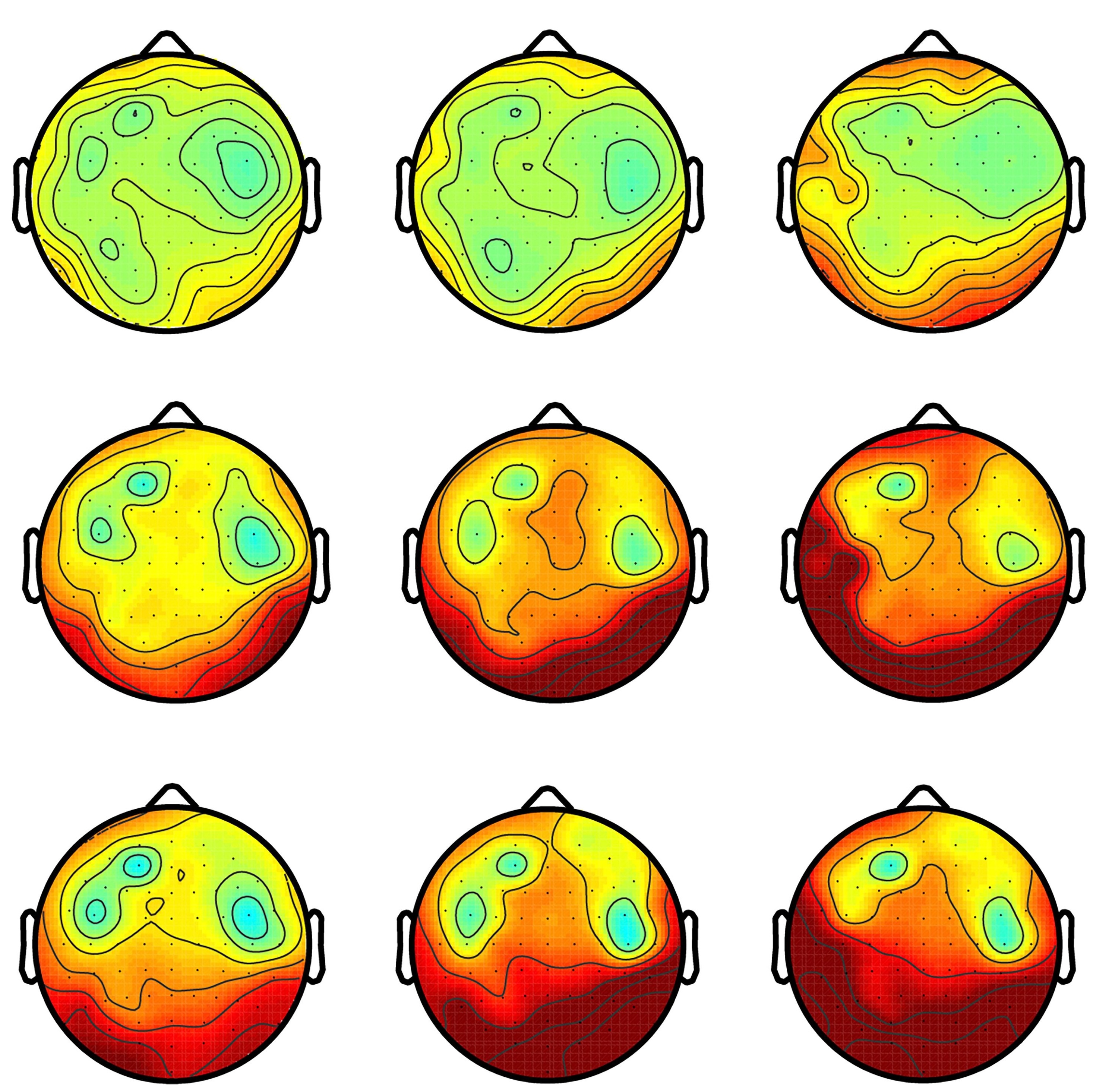
Data visualizations ↑
Further reading ↑
☆ Kasamatsu, A. & Hirai, T. (1966). An electroencephalographic study on the Zen meditation (zazen). Psychiatry and Clinical Neurosciences 20, 315–336. An extraordinary early study of the neural correlates of Zen meditation. Analyzing a sample of 48 Zen practitioners of varying experience and a control group of 22 volunteers—a large sample even by today’s standards of meditation research—the authors find that zazen is characterized by four stages: 1) the appearance of alpha waves, 2) an increase in alpha wave amplitude, 3) a decrease in alpha wave frequency, and 4) the arrival of a theta train. Experienced meditators were also more likely() to resist habituation to a click stimulus. These patterns were positively correlated with meditation experience. 10
☆Lutz, A., Greischar, L. L., Rawlings, N. B., Ricard, M., & Davidson, R. J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences, 101(46), 16369-16373. A very influential study showing that gamma oscillations, both absolute and as a ratio with slow waves (theta and alpha), may be the strongest signals of non-directed meditative states, such as those produced by loving-kindness meditation (and potentially zazen). 10
☆ Brewer, J. A., Worhunsky, P. D., Gray, J. R., Tang, Y. Y., Weber, J., & Kober, H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences, 108(50), 20254-20259. The first study to establish that key nodes of the default mode network (DMN), especially the posterior cingulate cortex and the medial prefrontal cortex, are relatively deactivated in meditators. Functional connectivity across brain regions was also enhanced among meditators. 9
☆ Tang, Y. Y., Hölzel, B. K., & Posner, M. I. (2015). The neuroscience of mindfulness meditation. Nature Reviews Neuroscience, 16(4), 213-225. An excellent overview of the field circa 2015. Tang et al. provide conceptual and physical maps of the relationship between brain functioning and meditation. The reference list serves as a comprehensive index. 9
Cahn, B. R., Delorme, A., & Polich, J. (2010). Occipital gamma activation during Vipassana meditation. Cognitive Processing, 11, 39-56. Careful work: a focus on a specific type of meditation, Vipassana; a nuanced description of results with respect to brain region; an exploration of confounders. The strongest results are a decrease in frontal delta and an increase in parieto-occiptal gamma power, with few state effects for the theta, beta, and alpha bands. The authors suggest the applicability of early alpha findings may be limited to novice meditators. 8
Aftanas, L. I., & Golocheikine, S. A. (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neuroscience letters, 310(1), 57-60. A theta-focused study of Sahaja Yoga meditation. Key conclusions include much greater theta and alpha-1 power in long-term versus short-term meditators, especially in anterior temporal and frontal regions; increased theta synchronization between (especially left) prefrontal region (AF3) and posterior association cortex; and more intense bliss feelings, and reduced thought appearance, correlated with increased theta power in anterior frontal and frontal midline areas and increased alpha-1 power in midcentral areas. Theta phenomena are possibly linked to focused attention, enhanced positive emotion, and reduced anxiety. 7
Banquet, J. P. (1973). Spectral analysis of the EEG in meditation. Electroencephalography and Clinical Neurophysiology, 35(2), 143-151. Another confirmation of Kasamatsu (alpha dominance, lowering in frequency and increasing in amplitude, followed by theta trains) with some additional observations on rhythmic beta waves among advanced meditators. 7
Buzsaki, G., & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304(5679), 1926-1929. A useful summary of electrical activity patterns in the mammalian brain, with presentation of possible functions of synchronized firing with given frequency bands. 7
Cahn, B. R., & Polich, J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological bulletin, 132(2), 180. A well-organized review of the effects of meditation on the brain. Theta and alpha power increase in amplitude, and alpha decreases in frequency, as one becomes more experienced in practice. Both trait (during practice) and state (long-term) impacts are present. More work is needed on distinguishing traditions, experience, and early-stage sleep markers. 7
Cahn, B. R., Delorme, A., & Polich, J. (2013). Event-related delta, theta, alpha and gamma correlates to auditory oddball processing during Vipassana meditation. Social cognitive and Affective Neuroscience, 8(1), 100-111. More sensitivity and awareness to stimuli, and decreased automatic reactivity, reflected in theta-phase consistency, early-event alpha synchronization, decreased early-evoked delta, and late-event alpha desynchronization. 7
Fayed, N., Lopez del Hoyo, Y., Andres, E., Serrano-Blanco, A., Bellón, J., Aguilar, K., … & Garcia-Campayo, J. (2013). Brain changes in long-term zen meditators using proton magnetic resonance spectroscopy and diffusion tensor imaging: a controlled study. PLoS One, 8(3), e58476. The first study to use magnetic resonance spectroscopy to look at the effects of long-term meditation on cerebral metabolism. Meditators have higher myo-inositol in the posterior cingulate gyrus and decreased glutamate, acetyl-asparate, and N-acetyl-asparate/creatine in the left thalamus. 7
Dor-Ziderman, Y., Berkovich-Ohana, A., Glicksohn, J., & Goldstein, A. (2013). Mindfulness-induced selflessness: a MEG neurophenomenological study. Frontiers in Human Neuroscience, 7, 582. Using a framework of narrative self (NS), minimal self (MS), and selfless (SL) subjectively experienced states, Dor-Ziderman et al. find that NS attentuation (i.e., transition to MS) is associated with high-gamma decreases in power, especially in the frontal, thalamic, and extensive dorsal and central mPFC regions; and MS attentuation (i.e., transition to SL) with beta band decreases in power, especially in the left ventral mPFC and thalamus. 6
Faber, P. L., Lehmann, D., Gianotti, L. R., Milz, P., Pascual-Marqui, R. D., Held, M., & Kochi, K. (2015). Zazen meditation and no-task resting EEG compared with LORETA intracortical source localization. Cognitive Processing, 16, 87-96. Zazen-specific study that finds increased alpha-1 and alpha-2 in the right hemisphere and decreased beta-1 and beta-1 activity. The former is hypothesized to be linked to internally directed attention, wakeful resting, and emotional processing; the latter to reduced focus on the visual stream. 6
Guidotti, R., Del Gratta, C., Perrucci, M. G., Romani, G. L., & Raffone, A. (2021). Neuroplasticity within and between functional brain networks in mental training based on long-term meditation. Brain Sciences, 11(8), 1086. An fMRI/multivariate pattern analysis mapping of active (and less active) networks during focused attention and open monitoring meditation. The networks differ, and their structure predicts both age and expertise. 6
Lutz, A., Jha, A. P., Dunne, J. D., & Saron, C. D. (2015). Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. American Psychologist, 70(7), 632. Lutz et al.’s ideas are helpful not because of their plausibility—it’s not at all clear that their functional dimensions are orthogonal; it’s easy to think of arguments for dereification leading to lowered object orientation, for example, or meta-awareness facilitating dereification—but because the exercise itself helps untangle some of the conceptual mess. There’s a lot of work to be done, and proposals that generate hypotheses are a critical part of the game. 6
Omori, M., Kosaka, H., Kikuchi, M., … & Wada, Y. (2005). Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. International Journal of Psychophysiology, 55(2), 199-207. Increases in fast theta and slow alpha in 20 inexperienced Zen meditators. Useful look at linkages to personality, including observed positive correlations with novelty seeking and harm avoidance. 6
Wallace, R. K. (1970). Physiological effects of transcendental meditation. Science, 167(3926), 1751-1754. Largely a confirmation of the results of Kasamatsu (1966) with a smaller and WEIRDer sample. 6
Anand, B. K., Chhina, G. S., & Singh, B. (1961). Some aspects of electroencephalographic studies in yogis. Electroencephalography and Clinical Neurophysiology, 13(3), 452-456. Cold water, strong light, loud banging, hot glass, tuning forks…nothing throws yogis off their alpha wave. An interesting early study, but the sample size (four yogis) leaves room for skepticism. 5
Davidson, R. J., & Dahl, C. J. (2018). Outstanding challenges in scientific research on mindfulness and meditation. Perspectives on Psychological Science, 13(1), 62-65. A reminder that the problems of meditation research are not new to psychology. “Mindfulness,” and even “meditation,” is not a well-defined construct. Research focuses on only a few forms of meditative practice. Many traditions indeed regard meditation as a balm for suffering, but not necessarily for the specific mental illnesses of today. Addressing individual differences and increasing sample sizes, especially through digital technology, is important. 5
Pagano, R. R., Rose, R. M., Stivers, R. M., & Warrenburg, S. (1976). Sleep during transcendental meditation. Science, 191(4224), 308-310. Sometimes you take a nap while meditating! That’s a good thing. When tired, sleep; when ready, sit. 3
Works Cited